Nitrogen Cycle Easy Diagram Nitrogen Cycle Diagram
Nitrogen cycle is an important part of the ecosystem. In this article, we shall explore its implications on the environment in detail.
Table of Contents
- What is Nitrogen Cycle
- Stages
- In Marine Ecosystem
- Importance
- Conclusion
Nitrogen Cycle Definition
"Nitrogen Cycle is a biogeochemical process which transforms the inert nitrogen present in the atmosphere to a more usable form for living organisms."
Furthermore, nitrogen is a key nutrient element for plants. However, the abundant nitrogen in the atmosphere cannot be used directly by plants or animals. Read on to explore how the Nitrogen cycle makes usable nitrogen available to plants and other living organisms.
What is the Nitrogen Cycle?
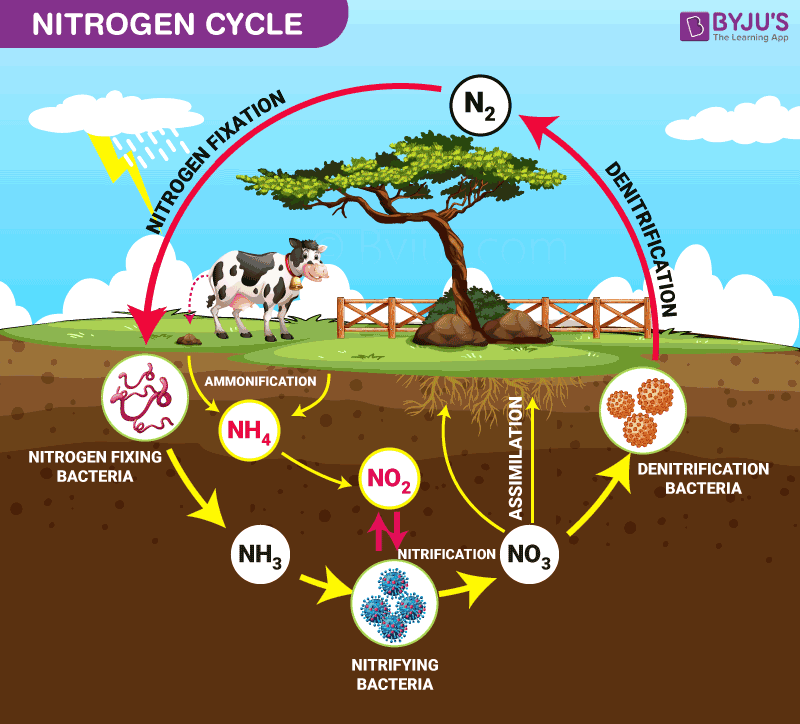
Nitrogen Cycle is a biogeochemical process through which nitrogen is converted into many forms, consecutively passing from the atmosphere to the soil to organism and back into the atmosphere.
It involves several processes such as nitrogen fixation, nitrification, denitrification, decay and putrefaction.
Nitrogen gas exists in both organic and inorganic forms. Organic nitrogen exists in living organisms, and they get passed through the food chain by the consumption of other living organisms.
Inorganic forms of nitrogen are found in abundance in the atmosphere. This nitrogen is made available to plants by symbiotic bacteria which can convert the inert nitrogen into a usable form – such as nitrites and nitrates.
Nitrogen undergoes various types of transformation to maintain a balance in the ecosystem. Furthermore, this process extends to various biomes, with the marine nitrogen cycle being one of the most complicated biogeochemical cycles.
Nitrogen Cycle Explained – Stages of Nitrogen Cycle
Process of the Nitrogen Cycle consists of the following steps – Nitrogen fixation, Nitrification, Assimilation, Ammonification and Denitrification. These processes take place in several stages and are explained below:
Nitrogen Fixation Process
It is the initial step of the nitrogen cycle. Here, Atmospheric nitrogen (N2) which is primarily available in an inert form, is converted into the usable form -ammonia (NH3).
During the process of Nitrogen fixation, the inert form of nitrogen gas is deposited into soils from the atmosphere and surface waters, mainly through precipitation.
The entire process of Nitrogen fixation is completed by symbiotic bacteria, which are known as Diazotrophs. Azotobacter and Rhizobium also have a major role in this process. These bacteria consist of a nitrogenase enzyme, which has the capability to combine gaseous nitrogen with hydrogen to form ammonia.
Nitrogen fixation can occur either by atmospheric fixation- which involves lightening, or industrial fixation by manufacturing ammonia under high temperature and pressure conditions. This can also be fixed through man-made processes, primarily industrial processes that create ammonia and nitrogen-rich fertilisers.
Recommended Video:
Types of Nitrogen Fixation
- Atmospheric fixation: A natural phenomenon where the energy of lightning breaks the nitrogen into nitrogen oxides, which are then used by plants.
- Industrial nitrogen fixation: It is a man-made alternative that aids in nitrogen fixation by the use of ammonia. Ammonia is produced by the direct combination of nitrogen and hydrogen. Later, it is converted into various fertilisers such as urea.
- Biological nitrogen fixation: We already know that nitrogen is not used directly from the air by plants and animals. Bacteria like Rhizobium and blue-green algae transform the unusable form of nitrogen into other compounds that are more readily usable. These nitrogen compounds get fixed in the soil by these microbes.
Also Read: Nitrogen Fixation And Nitrogen Metabolism
Nitrification
In this process, the ammonia is converted into nitrate by the presence of bacteria in the soil. Nitrites are formed by the oxidation of ammonia with the help of Nitrosomonas bacteria species. Later, the produced nitrites are converted into nitrates by Nitrobacter. This conversion is very important as ammonia gas is toxic for plants.
The reaction involved in the process of Nitrification is as follows:
2NH3 + 3O2 → 2NO2 – + 2H++ 2H2O
2NO2 – + O2→ 2NO3 –
Assimilation
Primary producers – plants take in the nitrogen compounds from the soil with the help of their roots, which are available in the form of ammonia, nitrite ions, nitrate ions or ammonium ions and are used in the formation of the plant and animal proteins. This way, it enters the food web when the primary consumers eat the plants.
Ammonification
When plants or animals die, the nitrogen present in the organic matter is released back into the soil. The decomposers, namely bacteria or fungi present in the soil, convert the organic matter back into ammonium. This process of decomposition produces ammonia, which is further used for other biological processes.
Denitrification
Denitrification is the process in which the nitrogen compounds make their way back into the atmosphere by converting nitrate (NO3-) into gaseous nitrogen (N). This process of the nitrogen cycle is the final stage and occurs in the absence of oxygen. Denitrification is carried out by the denitrifying bacterial species- Clostridium and Pseudomonas, which will process nitrate to gain oxygen and gives out free nitrogen gas as a byproduct.
Nitrogen Cycle in Marine Ecosystem
The process of the nitrogen cycle occurs in the same manner in the marine ecosystem as in the terrestrial ecosystem. The only difference is that it is carried out by marine bacteria.
The nitrogen-containing compounds fall into the ocean as sediments get compressed over long periods and form sedimentary rock. Due to the geological uplift, these sedimentary rocks move to land. Initially, it was not known that these nitrogen-containing sedimentary rocks are an essential source of nitrogen. But, recent researches have proved that the nitrogen from these rocks is released into the plants due to the weathering of rocks.
Importance of Nitrogen Cycle
The importance of the nitrogen cycle are as follows:
- Helps plants to synthesise chlorophyll from the nitrogen compounds.
- Helps in converting inert nitrogen gas into a usable form for the plants through the biochemical process.
- In the process of ammonification, the bacteria help in decomposing the animal and plant matter, which indirectly helps to clean up the environment.
- Nitrates and nitrites are released into the soil, which helps in enriching the soil with the necessary nutrients required for cultivation.
- Nitrogen is an integral component of the cell and it forms many crucial compounds and important biomolecules.
Nitrogen is also cycled by human activities such as the combustion of fuels and the use of nitrogen fertilisers. These processes increase the levels of nitrogen-containing compounds in the atmosphere. The fertilisers containing nitrogen are washed away in lakes, rivers and result in eutrophication.
Conclusion
- Nitrogen is abundant in the atmosphere, but it is unusable to plants or animals unless it is converted into nitrogen compounds.
- Nitrogen-fixing bacteria play a crucial role in fixing atmospheric nitrogen into nitrogen compounds that can be used by plants.
- The plants absorb the usable nitrogen compounds from the soil through their roots. Then, these nitrogen compounds are used for the production of proteins and other compounds in the plant cell.
- Animals assimilate nitrogen by consuming these plants or other animals that contain nitrogen. Humans consume proteins from these plants and animals. The nitrogen then assimilates into our body system.
- During the final stages of the nitrogen cycle, bacteria and fungi help decompose organic matter, where the nitrogenous compounds get dissolved into the soil which is again used by the plants.
- Some bacteria then convert these nitrogenous compounds in the soil and turn it into nitrogen gas. Eventually, it goes back to the atmosphere.
- These sets of processes repeat continuously and thus maintain the percentage of nitrogen in the atmosphere.
Further Reading: Other Biogeochemical Cycles
To explore more about the Nitrogen cycle, or the steps involved, keep visiting BYJU'S Biology website or download the BYJU'S app, for further reference.
Frequently Asked Questions
Why is nitrogen important for life?
Nitrogen constitutes many cellular components and is essential in many biological processes. For instance, the amino acids contain nitrogen and form building blocks that make up various components of the human body such as hair, tissues and muscles.
Why do plants need nitrogen?
Plants need nitrogen as this element is an important component of chlorophyll. Consequently, chlorophyll is vital for the process of photosynthesis, so lack of nitrogen can cause deficiency disorders such as stunted growth and other abnormalities
List the different steps that explain the Nitrogen Cycle process.
- Nitrogen Fixation
- Assimilation
- Ammonification
- Nitrification
- Denitrification
What is Ammonification?
Ammonification occurs during the decomposition of organic matter, where ammonifying bacteria convert organic nitrogen into inorganic components like ammonia or ammonium ions.
What is Nitrification?
Nitrification is a process that converts ammonia into nitrate by bacteria. Initially, the ammonia is converted to nitrite (NO2 −) by the bacteria Nitrosomonas, or Nitrococcus, etc., and then to nitrate (NO3 –) by Nitrobacter.
What is Denitrification?
Denitrification is the process of converting the nitrate back into molecular nitrogen by bacterias such asPseudomonas, Thiobacillus, Bacillus subtilis etc.
What is the function of nitrifying bacteria?
Nitrifying bacteria are a small group of aerobic bacteria, which are mainly involved in the conversion of ammonia into nitrates.
Which part of the plant is involved in nitrogen fixation?
The process of nitrogen fixation is carried out naturally in the soil within nodules in the plant's root systems.
Explore more topics – from photosynthesis and flowering plants to human anatomy and cryotechnology, only at BYJU'S Biology.
Source: https://byjus.com/biology/nitrogen-cycle/
0 Response to "Nitrogen Cycle Easy Diagram Nitrogen Cycle Diagram"
Post a Comment